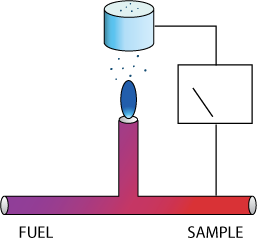
Flame ionization is a well-established measurement technique.
Combustible gases burned in a hydrogen flame produce ions that can be measured as a weak current through an imposed electric field. A well designed FID efficiently and completely burns the sample by pre-mixing a small quantity of sample with hydrogen fuel and injecting it into a burner, and then measures the resulting ion current. The signal is proportional to the weight of carbon present in the sample, except where the combustible gas contains oxygen, halogens, or in general other electronegative species, which tend to inhibit the formation of ions.
The response time of the detector is very fast, and in some cases the response factors are reasonably consistent. However, the need to inject a sample though a capillary tube can cause maintenance problems and significant loss of signal, and it is quite possible to have a high variation in response factors for some mixtures of different combustible gases.

Add new comment