Combustible gases absorb infrared radiation at certain characteristic wavelengths.
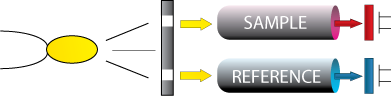
A typical non-dispersive infrared detector passes a pulsed source of infrared energy through the sample, and measures the energy received by two detectors.
One “active” detector responds to wavelengths in the same band as the combustible gas, and the other detector responds to wavelengths in a reference band to compensate for changes in the source.
When combustible gases are present, they absorb energy according to Beer’s Law, and produce a signal in the active detector relative to the reference detector. Beer’s Law states that the energy absorbed by the combustible gas for a given wavelength varies exponentially with the particular gas’s absorptivity, the concentration, and the path length.
This means that the infrared detector must be specifically designed for a particular gas, and can have very high variations in response factors and non-linearity for other gases and especially for mixtures. In practice this detector is often limited to use with a single combustible gas.

Add new comment