The Safety and Performance of your Printing Line: Sensor Choices and How to Decide
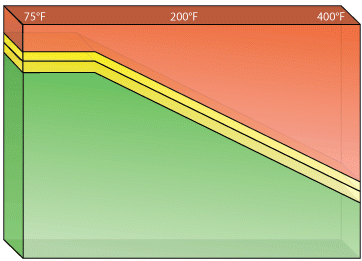
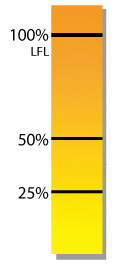
Although several different types of sensors are employed as LFL monitors, each has an appropriate application to which it is best suited.
Fires and explosions in what was thought to be “protected” equipment can occur without warning when a sensor is not capable of doing the job that had been assigned to it.
This is most often caused by a misunderstanding of the different available technologies.